Sleep study at home
Description:
Disturbed sleep is an early and modifiable risk factor for cognitive decline. The loss of slow-wave sleep (SWS), the deepest sleep stage, is linked to worsening memory function and reduced metabolic clearance, leading to a vicious cycle where cognitive decline and poor sleep reinforce each other. Enhancing SWS could help slow cognitive deterioration by supporting brain recovery.
This study explores whether phase-locked auditory stimulation (PLAS)—a technique that enhances slow-wave activity through gentle sound stimulation—can improve sleep, memory, and brain function. Previous laboratory research has shown that the brain’s response to PLAS correlates with improved memory performance and metabolic clearance, but larger home-based trials are needed.
The study aims to show that PLAS improves sleep, memory, and metabolic clearance, potentially restoring brain activity patterns seen in younger individuals. If successful, this research could support the development of affordable, non-invasive home-use solutions to help prevent cognitive decline.
Procedure:
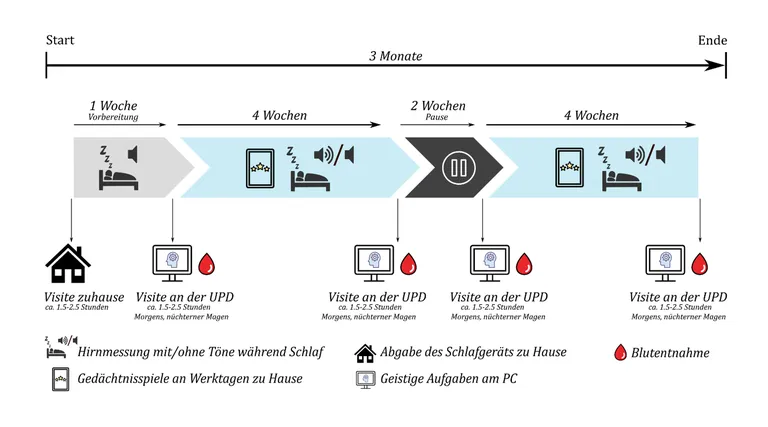
Adaptation phase
- Home visit: The experimenter visits participants to explain the study and provide study materials.
- Adaptation week: Participants use the sleep device nightly at home and engage in daily memory games on a tablet app to familiarize themselves with the procedure.
- Baseline assessment: After the adaptation week, participants attend an initial clinic visit for cognitive testing and blood sample collection.
First 4-week assessment period
- First 4-week intervention phase: Participants use the sleep device nightly at home and engage in daily memory games on a tablet app.
- Midpoint assessment: After 4 weeks, participants return to the clinic for cognitive testing and blood sample collection.
2- week break:
- 2-week break: Participants neither use the sleep device nor play the memory games during this period.
Second 4-week assessment period
- Pre-second intervention assessment: Before the second intervention phase, participants return to the clinic for cognitive testing and blood sample collection.
- Second 4-week intervention phase: Participants resume using the sleep device nightly and continue daily memory exercises on the tablet app.
- Final assessment: Participants attend a final clinic visit for cognitive testing and the last blood sample collection.
Eligibility criteria:
- Aged 60 to 85
- Experiencing mild memory difficulties
- No psychiatric or neurological disorders
- No hearing impairments or uncorrected vision problems
- No significant sleep issues or use of sleep medications
- Fluent in German
- Not living alone
Compensation:
Participants will receive 400 CHF upon full completion of the study.